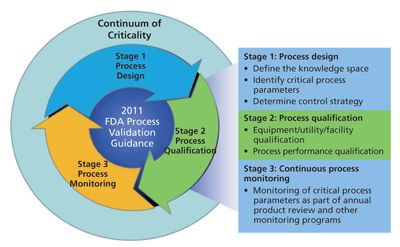
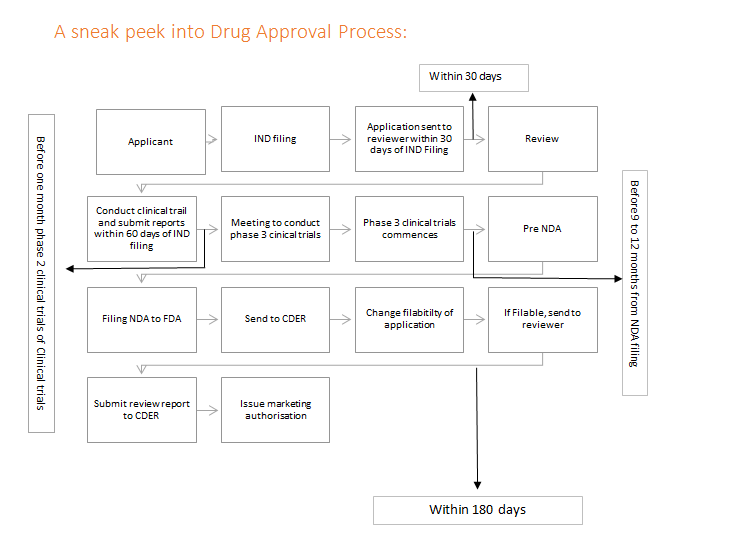
Ghtf study group 3 quality management systems process validation guidance january 2004 page 5 1 purpose and scope 1 1 purpose this process validation guidance is intended to assist manufacturers in understanding quality management system requirements concerning process validation.
Quality management system fda guidance.
The fda worldwide quality system requirements guidebook for medical devices.
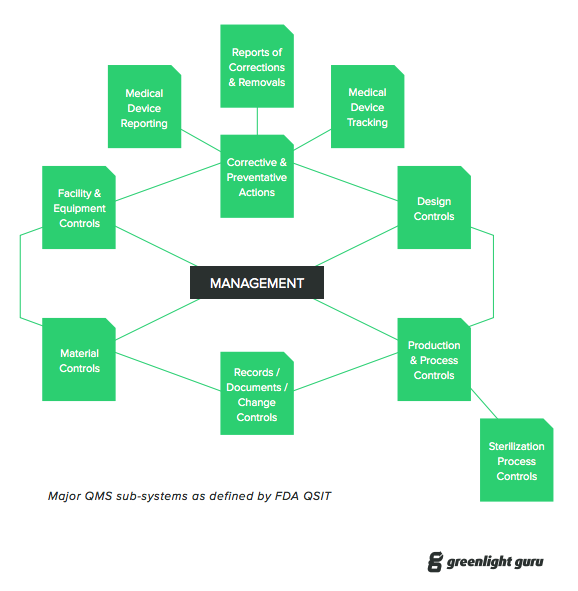
A quality management system qms is defined as a formalized system that documents processes procedures and responsibilities for achieving quality policies and objectives.
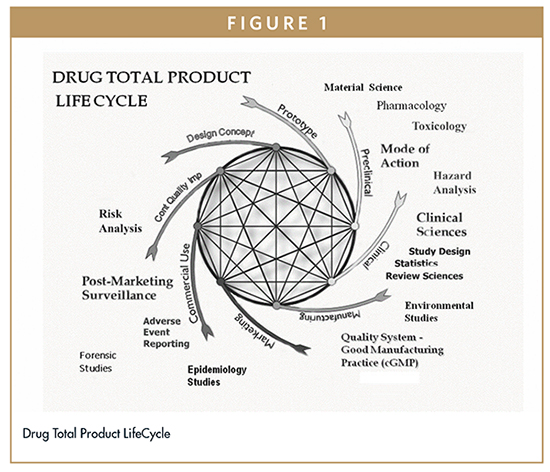
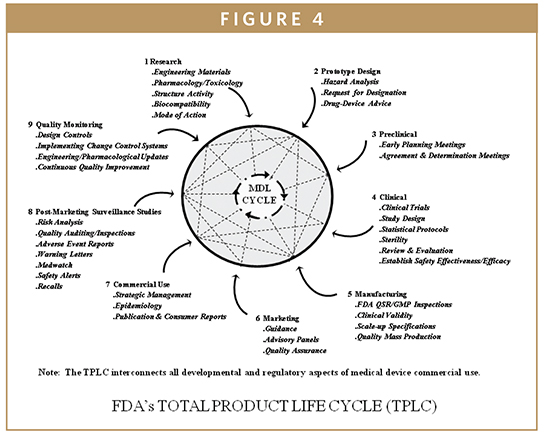
The quality system elements and management responsibilities described in this guidance are intended to encourage the use of science and risk based approaches at each.
This guidance serves as a.
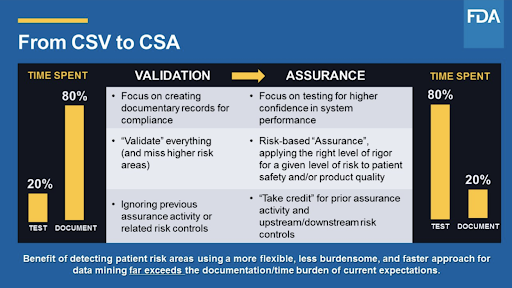
This new guidance will provide guidelines for streamlining documentation with an emphasis on critical thinking risk management patient and product safety data integrity and quality assurance p.
Quality system information for certain premarket application reviews guidance for industry and fda staff pdf 548kb design controls.
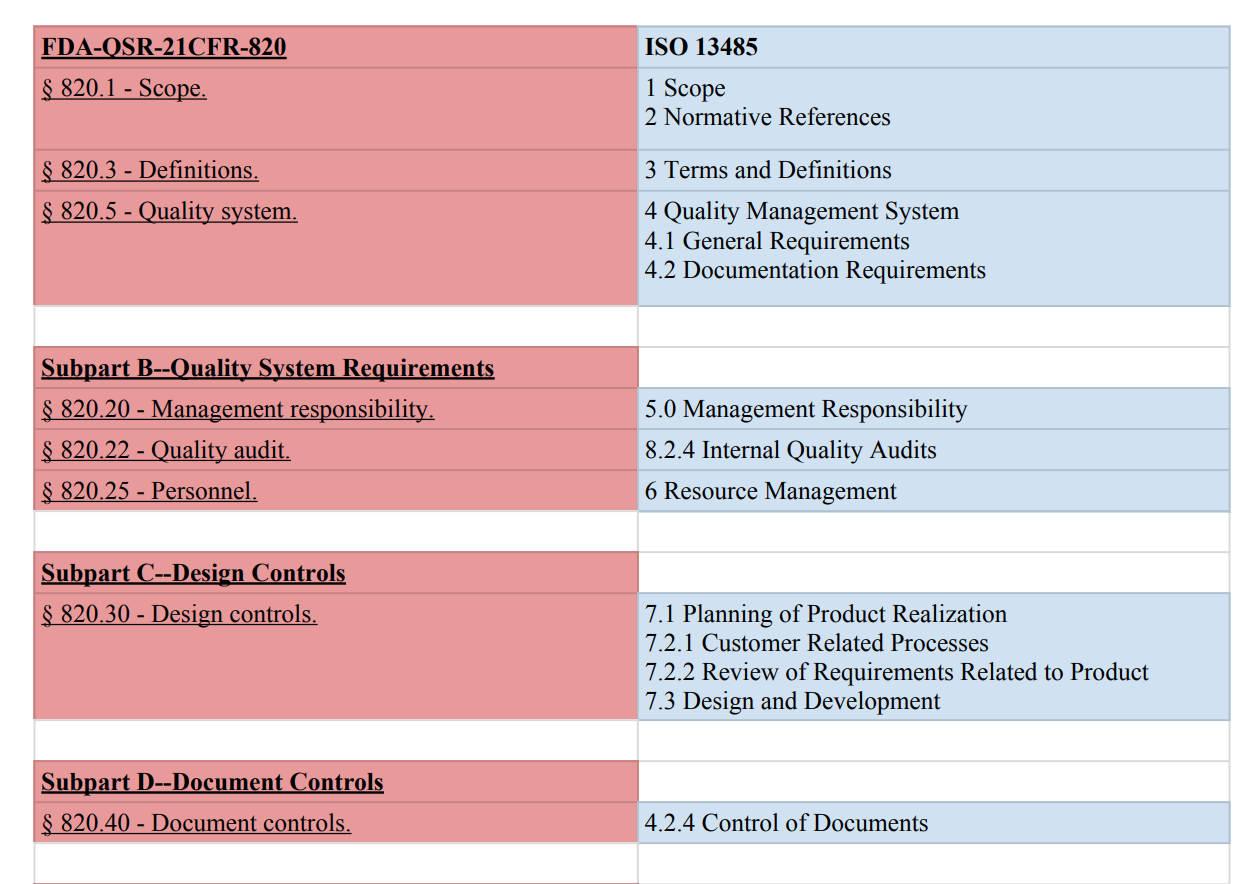
Pharmaceutical quality management requirements and fda s own medical device quality system regulations.
In addition there should be written versioned procedural documents sops.
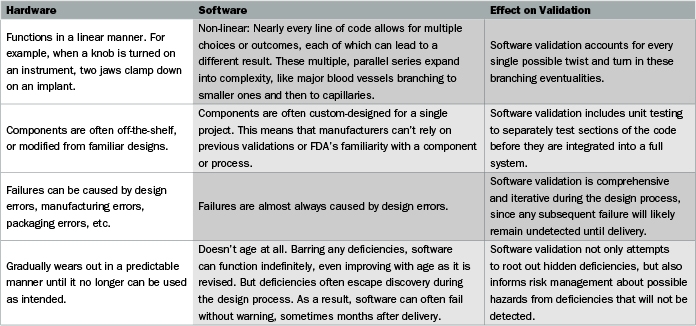
This guidance is intended to help manufacturers implementing modern quality systems and risk management approaches to meet the requirements of the agency s current good manufacturing practice.
A qms helps coordinate and direct an organization s activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous.
Each company should have a quality management system qms which includes a mission statement of the goals and scope of the program as well as defining applicable laws regulations and importantly best practices procedures where the law is unclear or silent.
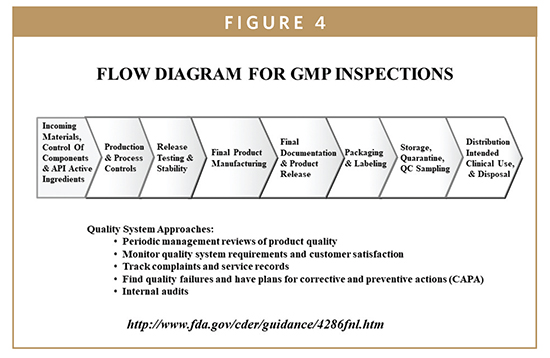
Other device specific guidance documents prepared by cdrh for the medical device industry.
Quality system regulation guidance documents.